Processing Lunar Soils for Oxygen and Other Materials
Christian W. Knudsen and Michael A. Gibson
Two types of lunar materials are excellent candidates for lunar oxygen production: ilmenite, FeTiO3, and silicates such as anorthite, CaAI2Si208 (Kesterke 1971, Williams et al. 1979, Williams and Erstfeld 1979, Steurer 1982, Carroll 1983). Both are lunar surface minable, occurring in soils, breccias, and basalts. Because silicates are considerably more abundant than ilmenite, they may be preferred as source materials. Depending on the processing method chosen for oxygen production and the feedstock material, various useful metals and bulk materials can be produced as byproducts. Available processing techniques include hydrogen reduction of ilmenite and electrochemical and chemical reductions of silicates (Williams and Jadwick 1980, Williams 1980. Processes in these categories are generally in preliminary development stages and need significant R&D support to carry them to practical deployment, particularly as a lunar-based operation.
The goal of beginning lunar processing operations by 2010 requires that planning and R&D emphasize the simplest processing schemes.. However, more complex schemes that now appear to present difficult technical challenges may offer more valuable metal byproducts later. While they require more time and effort to perfect, the more complex or difficult schemes may provide important processing and product improvements with which to extehd' and elaborate the initial lunar processing facilities. A balanced R&D program should take this into account.
Ilmenite- Process Semicontinuous
Primary hydrogen reduction of ilmenite is possible in a relatively clean reaction utilizing one-third of the contained oxygen (WiJliams and Erstfeld 1979):
![]()
Hydrogen would be imported from Earth and the resultant water would be electrolyzed to produce oxygen and recycle the hydrogen. If there are gaseous losses, hydrogen is perhaps the easiest material to make up since it must. be transported from Earth for peopulsion use.
The biggest limitation to this process is the low equilibrium conversion of this reaction. Relatively small water vapor pressures will stop the reduction and cause it to reverse. One way to enhance conversion has been proposed by Williams (1985). It involves using heat removal to reduce the water partial pressure by condensation in a cold trap, with the water partial pressure limited to its vapor pressure at trap temperature. As water is formed in the reactor, a water vapor pressure gradient is established, and water vapor diffuses from reactor to cold trap and condenses. If diffusion and heat removal rates are fast enough in relation to the water formation rate, the reactor water vapor pressure can be held lower than its equilibrium value at reactor temperature and this lower vapor pressure leads to a higher hydrogen conversion for a given reactor temperature. Oxygen would then be produced and hydrogen regenerated by electrolyzing the liquid water. Diffusion calculations indicate, however, that the vapor pressure cannot be lowered in a system with large enough dimensions to be practical.
Ilmenite - Continuous Fluid-Bed Reduction
Given the same ilmenite reduction reaction carried out in a reactor with continuous feeding of fresh ilmenite, withdrawal of spent ilmenite, and recirculation of hydrogen gas as both the fluidizing and the reducing medium, a continuous system can be conceived (see fig. 19) if a suitable high-temperature electrolyte can be found to continuously electrolyze the water vapor formed. Zirconium oxide stabilized with either calcium oxide or yttrium oxide appears to be a suitable electrolyte (Weissbart and Ruka 1962, Smith 1966). The high heat-transfer coefficients expected in this process between the gas and the fluidized solids would reduce the necessary size of the equipment. However, this scheme requires the retention of fine reactor solids and high- pressure hydrogen gas and the use of rotating machinery to circulate the hydrogen and to feed the solids. These requirements lead to operational complexities.
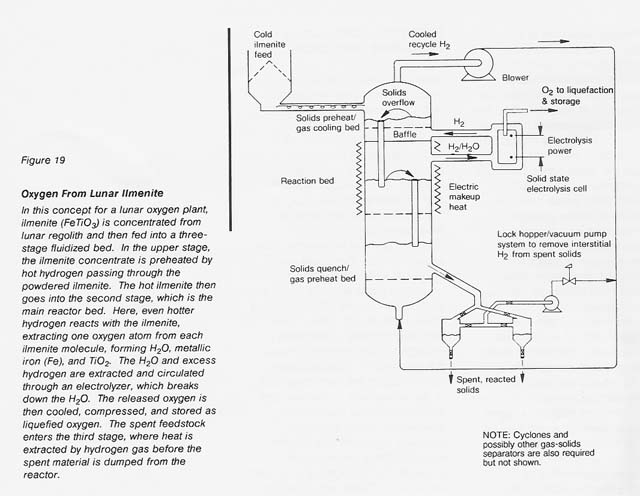
Utilization of Spent Ilmenite To Produce Bulk Materials
The hot, partially reduced ilmenite exiting the direct-reduction reactor might be pressed into blocks or bricks for use in structures or shielding. Further heating may be necessary i n the pressing operation. Evidence of successful hot pressing of brick-making shales and fireclays (Crayton and Brownell 1974) and of ashes from the combustion of coal mine wastes (Gartner 1979) into structurally strong blocks or bricks indicates that this utilization of spent ilmenite should be investigated.
Silicates- Electrochemical Reduction
In the case of silicates, hydrogen reduction is not thermodynamically favorable and proposed processing steps include direct electrochemical reduction, reduction with carbon plus chlorine, reduction with aluminum, and reduction with methane (Kesterke 1971; Carroll 1983; Colson and Haskin, immediately following; Williams and Erstfeld 1979; Anthony et al. 1988; Rosenberg et al., immediately preceding).
Kesterke studied direct electrochemical reductio~ to extract elemental oxygen from silicate- bearing melts experimentally. He used an iridium anode and LiF/BaF2 fluxing agents in a molten electrolyte/silicate bath at 1050 to 1250°C. Molecular oxygen was produced directly at the anode, but Kesterke found that fuming led to loss of volatile LiF from the electrolyte cell. More importantly, Kesterke concluded that the recovery of the fluoride fluxing agents from the spent electrolyte bath was prohibitively complex. Given experimental observation that SiO2. the major oxide constituent of Kesterke's melt, experienced little electroreduction, we conclude that large quantities of flux would have to be supplied from Earth or suitable substitutes found on the Moon near the facility.
In all of Kesterke's experiments, a solid deposit of varying size accumulate'd on the cathode. In this deposit, iron, aluminum, sodium, silicon, and barium were major constituents; manganese, titanium, calcium, molybdenum, and boron were present in significant quantities (the boron and molybdenum probably came from the experimental apparatus); and nickel, zirconium, copper, and chromium were present in lesser amounts. Iron would probably be the first metal produced from a lunar melt since it is the first major product of sequential electrolysis. Further electrolysis of the iron-free melt might yield other metals when processes are perfected.
Further work (including Carroll 1983) suggests that electrolysis techniques may be developed without fluxing materials and with a nonconsumable anode for oxygen production (see the paper immediately following by Colson and Haskin). Preliminary experiments indicate that molten rock or soil is conductive enough to support electrolysis.
Lunar application of electrolysis would yield oxygen directly as well as a number of important metals. Considerable improvement of electrode and containment materials are needed, as are lunar- derived fluxing materials for processes requiring a flux. Ultimately, development of a continuous or semicontinuous process would be desirable to provide uniform product quality and significant production rates.
Silicates-Chemical Reduction
The carbon-plus-chlorine silicate reduction process has several technical difficulties, as outlined in Williams and Erstfeld's thermodynamic study (1979). First, there are uncertainties about the numerous possible products of a high-temperature reaction of chlorine with complex silicates and carbon and about the ability to regenerate the chlorine and carbon quantitatively. Second, the oxygen must be extracted by high- temperature electrolysis of a CO/CO2 mixture, which will deposit carbon that will probably be hard to remove continuously. Finally, a solid electrolyte of stabilized zirconium oxide (the probable choice for the CO/CO2 electrolysis) is likely to be corroded severely to ZrCI4 when in contact with hot, chlorine-bearing\gases.
More recently (1988), Anthony and colleagues have developed a process that uses aluminum metal to reduce silicon in an anorthite-rich melt containing a flux (see fig. 20). The aluminum oxide is decomposed by electrolysis,producing oxygen and metallic aluminum, which is then recycled. Because anorthite contains abundant aluminum, excess aluminum is produced by the process and can be recovered either as a metal or as an alloy with silicon. Calcium or calcium oxide is subsequently removed from the melt, so the flux can be recycled.
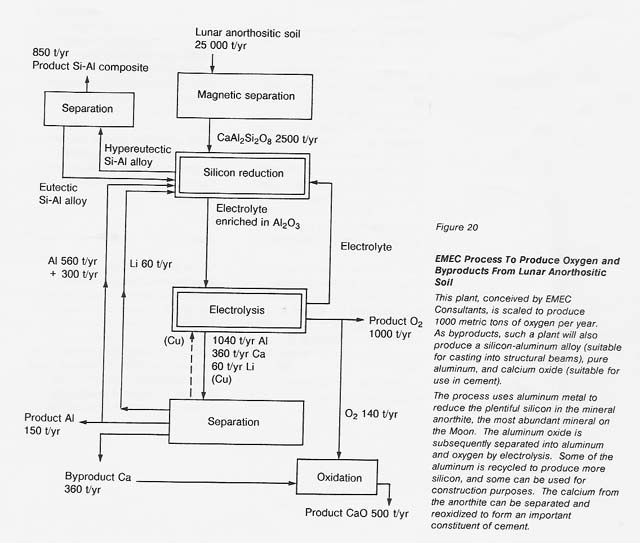
The EMEC process has the advantage that it can produce elemental silicon, elemental aluminum, and casting alloys of silicon and aluminum with low melting temperatures. Also, it can use most of the soil or rock as feedstock, if appropriate highland sites are chosen. This process has the disadvantage that it does require some feedstock beneficiation to eliminate most of the iron-rich minerals. Other disadvantages are that it must have inert electrodes that will not dissolve in the molten flux and it involves a somewhat complex step to recover the flux from the calcium oxide or calcium metal. However, the process is still attractive and the technology can borrow heavily from the existing aluminum-producing industry.
Carbothermal or methane reduction is another process that appears to have potential. It is proposed to operate at approximately 16250 C on molten silicates (Rosenberg et al., immediately preceding; see also NASA handout 1972). This process has the advantage of utilizing the whole soil, not just a beneficiated portion; thus, it requires the processing of a smaller amount of lunar soil. It does introduce corrosion problems. But perhaps the biggest challenge is the complexity and large heat exchange surface required because the methane must be regenerated at about 250°C by hydrogen reduction of carbon monoxide. Exothermic heat from this lower temperature step cannot be used to drive the primary reduction, which proceeds only at much higher temperatures. Nevertheless, methane reduction is clearly a viable candidate among the various chemical reduction processes proposed for silicates, especially if a use for lower level heat is available.
Summary
Of the processes discussed, ilmenite reduction appears to offer the most straightforward chemistry, the lowest operating temperature, the least materials problems, and the easiest to replicate working fluid. Its development needs to be pursued, but other processes need to be advanced simultaneously to develop a variety of processing routes and product possibilities.